 Our immune system is an intricate system of defenses that distinguishes between foreign substances within the body from healthy cells, when properly functioning. Nearly 90% of people see their immune system as their body’s most specialized defense mechanism in preventing illness, making the idea of “immune boosting” an appealing concept. It sounds simple and desirable. Can you boost immunity? So what Can You Do? It’s a completely invalid analogy.
Our immune system is an intricate system of defenses that distinguishes between foreign substances within the body from healthy cells, when properly functioning. Nearly 90% of people see their immune system as their body’s most specialized defense mechanism in preventing illness, making the idea of “immune boosting” an appealing concept. It sounds simple and desirable. Can you boost immunity? So what Can You Do? It’s a completely invalid analogy.
Attempting to boost the cells of the immune system is especially complicated because there are so many different kinds of cells in the immune system that respond to so many different microbes in so many ways. Which cells should you boost, and to what number? So far, scientists do not know the answer. There is still so much that they do not know about the intricacies and interconnectedness of the immune response. The major components of the immune system include lymph nodes, spleen, bone marrow, lymphocytes, thymus, and leukocytes. Our immune system is controlled by hormones secreted by the brain, thymus, adrenals and other glands, which regulate the production and activity of the many types of immune cells.
Rather than boosting, however, new science demonstrates that the immune system should be fine-tuned and requires balance and harmony. If your immune system is compromised or otherwise weakened, your body becomes more easily susceptible to infection. Chronic or frequent bacterial, viral or yeast infections may be signs of reduced immunity. In humans, immunodeficiency can either be the result of a genetic disease such as severe combined immunodeficiency, acquired conditions such as HIV/AIDS, or through the use of immunosuppressive medication.
Conversely, if your immune system is overactive, the immune system attacks your own healthy tissues. This is what we call autoimmune diseases. They are all caused by the immune system attacking different organs of the body. A chronic inflammatory disease is a medical condition which is characterized by persistent inflammation. Patients develop a chronic inflammatory disease because the immune system has an inappropriate response to something it has been exposed to. In some cases, this means that the patient develops an autoimmune disease, in which the immune system starts to attack the body. In other instances, the patient experiences chronic inflammation in response to certain foods or environmental factors such as smoke and chronic exposure to air pollution.
Conditions like lupus, rheumatoid arthritis, inflammatory bowel disease (ulcerative colitis, Crohn’s disease), Behcet’s disease, thyroid disease, psoriasis, atopic dermatitis, autoimmune pemphigus, scleroderma, celiac disease, type 1 diabetes, aphthous stomatitis, alopecia areata, autoimmune hemolytic anemia, idiopathic thrombocytopenic purpura, polyarteritis nodosa, autoimmune kidney disease, autoimmune hepatitis, primary sclerosing cholangitis, idiopathic pulmonary fibrosis, fibromyalgia syndrome, myasthenia gravis, ankylosing spondylitis, CIDP, multiple sclerosis, SjÖgren Syndrome, Guilian-Barre syndrome, Meniers disease (hearing loss, vertigo) autoimmune chronic fatigue syndrome, etc are all autoimmune diseases caused by an incorrect or overreaction of the immune system.
Since all autoimmune diseases use the same mechanism of action to attack target organs thus their treatment is essentially the same. However, achieving and maintaining immune balance can be a challenge in today’s world. Toxins, environmental hormones (endocrine disruptors), lack of sunlight , physical inactivity, lack of sleep, stress, financial strain, packed schedules, poor diet, overuse or inappropriate use of drugs like NSAID and antibiotics, intestinal dysbiosis (imbalanced gut flora), mutating viruses, antibiotic resistant bacteria, mold exposure, and more—all of these can wear you down. They can also compromise your immune system. Our modern lifestyle also appears to be linked to increases in autoimmune diseases, chronic inflammatory diseases, allergies, and cancers.
According to the following article, more women suffer from autoimmune diseases than men. Three quarters of suffers are women. Some suspected reasons may be genetics, sex hormones, and that women have more sophisticated immune systems with elevated antibody responses. Estrogen is known to stimulate the immune system while androgens are recognized to be immunosuppressive. The gut mucosa is a site of interaction between the gut microbiome and the host immune system. The involvement of the GI tract is well documented in several inflammatory and autoimmune disorders, including Crohn’s disease, ulcerative colitis, and Celiac disease. It is now known that the regulation of intestinal inflammatory response is influenced by sex differences, and baseline gut mucosal gene expression shows increased immune activation and inflammation in women compared to men.
Meanwhile, immune dysregulation is very common in major depression patients, with these individuals incurring increased risk for development of chronic inflammatory disease or autoimmune disease. Depression affects both men and women, but more women than men are likely to be diagnosed with depression. Scientists are examining many potential causes for and contributing factors to women’s increased risk for depression. It is likely that genetic, biological, chemical, hormonal, environmental, psychological, and social factors all intersect to contribute to depression.
In fact, there is extensive bi-directional communication between the immune system and the brain in both health and disease. Our immune system engages the brain in an intricate dialogue that can influence our thought processes, coaxing our brains to work at their best. T cells, white blood cells that are a key part of the immune system, may also play an important role in brain function. An imbalance between pro-inflammatory (IL-1β, IL-6, and TNF-α) and anti-inflammatory (IL-10) cytokines may play an important role in the pathogenesis of both depression and autoimmune disease.
Women and autoimmune diseases.
Genetic and hormonal factors in female-biased autoimmunity.
The role of gender and organ specific autoimmunity.
Sex differences matter in the gut: effect on mucosal immune activation and inflammation.
Interplay between pro-inflammatory cytokines and growth factors in depressive illnesses.
Depression and immunity: inflammation and depressive symptoms in multiple sclerosis.
Depression, another autoimmune disease from the view of autoantibodies.
Evidence for a Dysregulated Immune System in the Etiology of Psychiatric Disorders.
Inflammation is the first response of the immune system to infection or irritation. Without inflammation, we won’t be able to survive in a hostile world infested with dangerous microorganisms. However, inflammation is a hot topic in modern medicine. It appears connected to almost every known chronic disease-from allergies to asthma, diabetes to obesity, heart disease to cancer, autism to dementia, and even depression and all autoimmune diseases. Autoimmune diseases are ranked number one cause of heart disease, cancer and all diseases. It is also well established that most cancers develop at sites of chronic inflammation.
Pro-inflammatory T cells known as Th17 cells, pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α, and transcription factors such as NF-κB and STAT-3 are intrinsically involved in the initiation and progression of almost all autoimmune diseases. If we can control the activation of these pro-inflammatory factors, all autoimmune diseases will cease to exist. It’s that simple.
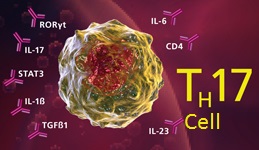 Th17 cells play an important role in host defense against bacterial and fungal infection, especially at mucosal surfaces. Th17 cells are localized primarily in tissues that separate the host from the environment, principally the skin and mucosa. Through their activation and subsequent cytokine production, they trigger pro-inflammatory signaling that promotes neutrophil mobilization and the expression of antimicrobial peptides, such as Reg3 gamma. Because of their role in inflammation, Th17 cells are implicated in a broad array of inflammatory and autoimmune responses. They play critical roles in autoimmune diseases such as rheumatioid arthritis, IBD (inflammatory bowel diseases), asthma, multiple sclerosis, psoriasis and many others.
Th17 cells play an important role in host defense against bacterial and fungal infection, especially at mucosal surfaces. Th17 cells are localized primarily in tissues that separate the host from the environment, principally the skin and mucosa. Through their activation and subsequent cytokine production, they trigger pro-inflammatory signaling that promotes neutrophil mobilization and the expression of antimicrobial peptides, such as Reg3 gamma. Because of their role in inflammation, Th17 cells are implicated in a broad array of inflammatory and autoimmune responses. They play critical roles in autoimmune diseases such as rheumatioid arthritis, IBD (inflammatory bowel diseases), asthma, multiple sclerosis, psoriasis and many others.
Th17 cells are a novel class of helper CD4+ T cells that secrete inflammatory cytokines such as IL-17A, IL-17F, IL-21, IL-22, IL-26 (human) and TNF alpha. Differentiation of naïve T cells towards a Th17 phenotype is supported by several cytokines including TGF-β, IL-1β, IL-6, IL-21, and IL-23. IL-23 is required for Th17 expansion and stabilization. IL-23, as well as TNF-α, acts as a survival signal for Th17 cells. The current consensus is that IL-6 induces Th17 differentiation together with TGF- β. It has been reported that IL-21, similar to IL-6, can also initiate Th17 differentiation combined with TGF- β.
STAT3 is critical for Th17 differentiation. STAT3 is also necessary for the expression of many transcription factors involved in Th17 differentiation. The inhibition of STAT3 activation can block Th17 activation while promoting Th1 (cell mediated) immune responses. Cytokines such as IFN-gamma, IL-27 and IL-4 are known to inhibit Th17 differentiation. IL-27 is also involved in regulating the balance between pro- and anti-inflammatory T cell responses. The pathogenic potential of Th17 cells are restrained by the co-production of IL-10. When Th17 cells express T-bet, and cease IL-10 production, they attain stronger pathogenic function. Paradoxically, the pro-inflammatory cytokine IL-6 and the anti-inflammatory cytokine IL-10 both activate STAT3, yet generate nearly opposing cellular responses.
Th1 and Th17 cells: adversaries and collaborators.
Th17 response and inflammatory autoimmune diseases.
increased Th17 response to pathogen stimulation in patients with primary sclerosing cholangitis.
The important role of T cells and receptor expression in Sjögren’s syndrome.
The serum IL-6 profile and Treg/Th17 peripheral cell populations in patients with type 1 diabetes.
The autoimmunity in Graves’s disease.
Transcription factors and th17 cell development in experimental autoimmune encephalomyelitis.
Autoimmune Memory T Helper 17 Cell Function and Expansion Are Dependent on Interleukin-23.
The pathogenic role of interleukin-27 in autoimmune diabetes.
TNF (tumor necrosis factor) activates the immune response against pathogens, including cancer cells. The TNF/TNF receptor (TNFR) system has a prominent role in the pathogenesis of chronic inflammatory and autoimmune diseases. TNF is usually overproduced in chronic inflammatory and autoimmune diseases, such as rheumatoid arthritis and multiple sclerosis.
IL-6 is released in response to inflammation or infection, and in turn stimulates the production of C-reactive protein (CRP) produced in the liver. The level of CRP rises when there is inflammation throughout the body. Elevated levels of CRP and IL-6 predicted the risk of many diseases, including cardiovascular disease, osteoporosis, type 2 diabetes, periodontal disease, frailty, Alzheimer’s disease, functional decline, muscle cell breakdown, lymphoid cancers like multiple myeloma, and certain cancers. Moreover, IL-6 makes significant contributions to such autoimmune and inflammatory diseases such as rheumatoid arthritis.
Unfortunately, TNF, IL-1 and IL-6 also act as crucial mediators of inflammation-driven tumorigenesis. NF-κB and STAT-3 also interact at multiple levels and thereby boost tumor-associated inflammation which can suppress anti-tumor immune responses. The protein p53 is the most important tumor suppressor in the body. Inactivation of the p53 tumor suppressor is a frequent event in tumorigenesis. Mutations in p53 are very common in many cancers (more than 50% of all cancers), which contribute to the longevity of the cancer cells. When a cell is stressed, p53 activates a wide number of pathways that promote apoptosis. P53 also induces apoptosis in virally infected cells. Interestingly, recent study shows that p53 activity in T cells suppresses autoimmunity by controlling Th17 effectors and thereby inhibits autoimmune inflammation. Under inflammatory conditions, p53 also suppresses Th17 cell differentiation. Selenium is known to activate p53. So far, we have two more natural agents that can promote p53 activity.
TNF superfamily in inflammatory disease: translating basic insights.
Cellular mechanisms of TNF function in models of inflammation and autoimmunity.
Interleukin 6 in autoimmune and inflammatory diseases: a personal memoir.
Chronic inflammation in cancer development.
TNF: a tumor-suppressing factor or a tumor-promoting factor?
TNF-dependent signaling pathways in liver cancer: promising targets for therapeutic strategies?.
Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer.
NF-κB and STAT3 – key players in liver inflammation and cancer.
Stat3: linking inflammation to (gastrointestinal) tumourigenesis.
Trp53 negatively regulates autoimmunity via the STAT3-Th17 axis.
Selenium and sulindac are synergistic to inhibit intestinal tumorigenesis in Apc/p21 mice.
Adenosine is an endogenous purine nucleoside that modulates many physiological processes. Adenosine is formed inside cells or on their surface. Cellular signaling by adenosine occurs through four known adenosine receptor subtypes (A1, A2A, A2B, and A3). Adenosine is an anti-inflammatory agent at the A2A receptor. A2A activation inhibits the NF-kB pathway and diminished inflammatory cytokines such as TNF-α, IL-1β and IL-6. The anti-inflammatory cytokine IL-10 plays a fundamental role in the development of immunological tolerance and the inhibition of inflammatory responses. Activation of both the A2A and A2B adenosine receptors promotes the synthesis of IL-10. Adenosine, via the activation of the A2A receptor, can also block the development of these dangerous Th17 immune responses. Clearly, adenosine is a major natural feedback molecule for the control of inflammation. Caffeine inhibits the activity of all four adenosine receptors. Caffeine does not cause autoimmune diseases, but it can strongly promote their development. If you have an autoimmune disease, caffeine should be excluded from the diet.
Adenosine promotes alternative macrophage activation via A2A and A2B receptors.
Adenosine, an endogenous distress signal, modulates tissue damage and repair.
High-salt diets can also lead to a dramatic induction of Th17 cells in a specific cytokine milieu. Recent studies suggest that salt may play a previously unknown role in triggering autoimmune diseases such as multiple sclerosis or type1 diabetes in individuals who are already genetically predisposed. The link between Th17 cell activity and salt is a new line of evidence that probes the relationship between autoimmune diseases and environmental factors such as diet.
Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells.
Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1.
Glycogen synthase kinase-3 (GSK-3) is a cytoplasmic serine/threonine protein kinase that phosphorylates and inhibits glycogen synthase, thereby inhibiting glycogen synthesis from glucose. GSK-3 regulates numerous cellular processes, and is also a critical mediator of Th17 cell production. GSK-3 inhibitors can control Th17-mediated diseases. Lithium inhibits GSK-3 activity both directly and indirectly. Interaction between magnesium and GSK-3 allows GSK-3 to phosphorylate many substrates. Magnesium ions are important for the binding of ATP (substrate) in the active site of GSK-3. In the presence of lithium, magnesium is unable to interact with the active site of GSK-3. In an alternate, indirect way, lithium increases the inhibitory phosphorylation of a critical serine residue in GSK-3, causing its inactivation. Lithium also modulates IFN-gamma signaling. IFN-gamma is associated with a number of autoimmune diseases.
Glycogen synthase kinase-3 is an early determinant in the differentiation of pathogenic Th17 cells.
Regulation by glycogen synthase kinase-3 of inflammation and T cells in CNS diseases.
Regulation of Th1 cells and experimental autoimmune encephalomyelitis by glycogen synthase kinase-3.
Inhibition of GSK3 by lithium, from single molecules to signaling networks.
Lithium controls central nervous system autoimmunity through modulation of IFN-γ signaling.
Th17 response in an autoimmune disease is associated with the appearance of CD25+ dendritic cells. Retinoic Acid, a lipophilic molecule and a metabolite of Vitamin-A, inhibits CD25+ dendritic cells.
Berberine is a natural alkaloid found in a wide variety of traditional herbs including goldenseal, barberry and Oregon grape. It is widely used as an anti-inflammatory remedy. Berberine suppresses Th17 responses. EGCG from green tea also inhibits Th17 differentiation.
Berberine suppresses Th17 and dendritic cell responses.
Green tea EGCG, T cells, and T cell-mediated autoimmune diseases.
Both chronic stress and age increase the level of IL-6 and TNF-α. Worse, the effects of stress and age are interactive. Psychological stress can both mimic and exacerbate the effects of aging, with older adults often showing greater immunological impairment to stress than younger adults. As compared to young adults, middle aged and particularly elderly adults typically have higher levels of cytokines with pro-inflammatory functions circulating in their blood, such as IL-6 and TNF-α.
Chronic stress stimulates cellular production of IL-6 and TNF-α along with impairments in the capacity of glucocorticoids (a class of steroid hormones) to inhibit this cellular inflammatory response. Chronic stress leads to chronic inflammation linked to obesity, depression, anxiety, insomnia, muscle pain, high blood pressure, heart disease, and dysregulated immune responses such as autoimmune disease. Both chronic stress and major depression are much bigger contributors to autoimmune and chronic inflammatory diseases than most of us really realize. There is a definite relationship between ongoing stressful life events before the onset of autoimmune disease.
Chronic stress and age-related increases in the proinflammatory cytokine IL-6.
Stress, age, and immune function: toward a lifespan approach.
Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk.
Immune balance is the ‘neutral gear’ of immune health that allows the body to respond most appropriately to the need to either activate or suppress immune function as needed. Our body’s immune system is multidimensional, it needs multidimensional support. All aspects of our life experiences, including spiritual, emotional, nutritional and environmental factors influence immune balance.
Immune modulation is a dynamic process that requires constant support. Unlike other immune supporting formulas out there in the marketplace, Hepazym is a clinically proven immune modulator that balances the function of your immune system. It has been developed in research on viral pathogenesis and mechanistic insights into viral modulation of immune receptor signaling. Hepazym contains the latest research based formula of bio-active fermented 32 naturally-occurring compounds with therapeutic potential in autoimmune and chronic inflammatory diseases based on modulating the immune response.
Hepazym formulas combine ancient wisdom with modern science to provide multidimensional support for your immune system. Hepazym contains a proprietary blend of organic wild mushrooms extracts, polysaccharides, 1-3, 1-6 beta glucans, immune molecules, botanical extracts, antioxidants, ionic plant based minerals, plant enzymes, probiotics, beneficial intestinal bacteria and other specially selected micronutrients to provide broad spectrum immune modulation via multiple pathways- immune cell function support, immune cell metabolism support, methylation support and antioxidant support. Hepazym is the most powerful immune modulator for the treatment of autoimmune and chronic inflammatory diseases. There is no more thoroughly tested for many years before they become available to patients in clinical trials and proven effective product on the market today.