The roles of zinc in the body are numerous. Among other things, it serves to boost immune functions and plays a significant role in healing processes and the development of healthy bones. Lesser known, but equally essential roles include helping with the formation of hundreds of enzymes, and assisting with gene transcription and RNA and DNA metabolism. It’s found in the tissues of the brain, kidneys, muscles, liver and bones; however the highest concentration is found in the eye and the male prostate gland. Total zinc levels in the prostate are 10 times higher than in other soft tissues. It is known to be crucial to normal prostate function.
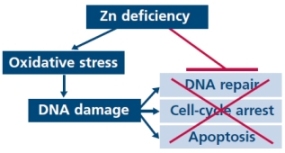 Zinc is essential to protecting against oxidative stress and helping DNA repair. Zinc deficiency increases oxidative stress, and DNA damage. It is now well established that zinc level is markedly decreased in malignant versus normal prostate tissue and, thus, prostate cancer cells are unable to accumulate zinc in high levels. Prostate is a unique organ that produces and releases large amounts of citrate. Citrate and iron are essential for the metabolism of most organisms, and regulation of citrate and iron biology at both the cellular and systemic levels is critical for normal physiology and survival.
Zinc is essential to protecting against oxidative stress and helping DNA repair. Zinc deficiency increases oxidative stress, and DNA damage. It is now well established that zinc level is markedly decreased in malignant versus normal prostate tissue and, thus, prostate cancer cells are unable to accumulate zinc in high levels. Prostate is a unique organ that produces and releases large amounts of citrate. Citrate and iron are essential for the metabolism of most organisms, and regulation of citrate and iron biology at both the cellular and systemic levels is critical for normal physiology and survival.
 In prostate cancer, normal citrate-producing glandular secretory epithelial cells undergo a metabolic transformation to malignant citrate-oxidizing cells. Mitochondrial aconitase (m-aconitase) is the critical step involved in this altered citrate metabolism that is essential to prostate malignancy, and it is possible that citrate is (re)taken up and used as a metabolite to enhance cellular activity. Furthermore, as zinc is normally an inhibitor of citrate oxidation, the reduction of zinc in prostate cancer may cause a decrease in citrate secretion levels in the glandular epithelia of prostate cancer patients.
In prostate cancer, normal citrate-producing glandular secretory epithelial cells undergo a metabolic transformation to malignant citrate-oxidizing cells. Mitochondrial aconitase (m-aconitase) is the critical step involved in this altered citrate metabolism that is essential to prostate malignancy, and it is possible that citrate is (re)taken up and used as a metabolite to enhance cellular activity. Furthermore, as zinc is normally an inhibitor of citrate oxidation, the reduction of zinc in prostate cancer may cause a decrease in citrate secretion levels in the glandular epithelia of prostate cancer patients.
The prevention of citrate oxidation by the prostate cells is the key relationship that is responsible for net citrate production. This metabolic effect has implications in altering energy metabolism and ATP (adenosine triphosphate) production of prostate cells, such that lower zinc levels in prostate cells leads to a higher rate of citrate oxidation, which increases the available energy and has been proposed to contribute to carcinogenesis and tumor growth.
A genetic alteration in the expression of ZIP1 zinc transporter is associated with this metabolic transformation. The intracellular level of all cells is firstly dependent upon the existence of zinc uptake transporters to extract zinc from external (e.g. interstitial fluid) sources. These genetic/metabolic relationships have important consequences on citrate-related metabolism, bioenergetics, cell proliferation and invasive capabilities of the malignant cells. In contrast to these highly specialized prostate cells, most mammalian cells cannot survive if m-aconitase activity and citrate oxidation are inhibited.
Zinc deficiency alters DNA damage response genes in normal human prostate epithelial cells.
Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland.
Zinc is decreased in prostate cancer: an established relationship of prostate cancer!
Metabolic regulation of citrate and iron by aconitases: role of iron-sulfur cluster biogenesis.
Mitochondrial aconitase and citrate metabolism in malignant and nonmalignant human prostate tissues.
Zinc blocks gene expression of mitochondrial aconitase in human prostatic carcinoma cells.
Zinc as an anti-tumor agent in prostate cancer and in other cancers.
Zinc deficiency is very common throughout the world. Age-related declines in immune function are similar to those associated with zinc deficiency, and the elderly are vulnerable to mild zinc deficiency. While the most common cause of deficiency is diet-related, conditions such as liver and renal disease, diabetes, sickle cell disease and many others can also lower zinc levels substantially by inhibiting absorption. The effects of chronically low zinc levels in the body may include impotence, suppressed sexual maturation, depression, skin lesions, loss of cognitive ability, hair loss, impaired immunity and several other conditions. Zinc deficiency is also an important factor in the development and progression of malignancy and that zinc could be efficacious in the prevention and treatment of several cancers viz., prostate, colon, pancreas, oesophageal, and head and neck.
Zinc deficiency causes significant impairment in both adaptive and innate immune responses, and promotes systemic chronic inflammation which then may increase the risk of developing inflammation-mediated diseases and cancer. It is extremely well established that inflammatory conditions in selected organs increase the risk of cancer. An inflammatory component is present also in the microenvironment of tumors that are not epidemiologically related to inflammation.
Metabolic factors and androgens may promote prostate carcinogenesis via multiple mechanisms including zinc deficiency, adipokine action, chronic inflammation, citrate metabolism, fatty acid metabolism and insulin growth factor signalling. Thus, treatment of chronic inflammation may represent an important therapeutic target in advanced prostate cancer.
Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability.
Zinc and its role in age-related inflammation and immune dysfunction.
Obesity, metabolic syndrome, and prostate cancer.
Are Toll-like receptor gene polymorphisms associated with prostate cancer?
Prostate cancer and inflammation: the evidence.
Advanced prostate cancer: reinforcing the strings between inflammation and the metastatic behavior.
NF-κB (nuclear factor kappa B) is a key orchestrator of innate immunity/inflammation. NF-kB regulates expression of multiple genes involved in tumor growth, metastasis and angiogenesis. A20 is a zinc finger protein and A20 gene is induced by NF-kB. A20 is a critical negative regulator of NF-κB and inflammation. This is a feedback response which prohibits a prolonged NF-kB response in cells, since A20-deficient mice develop uncontrolled and spontaneous multi-organ inflammation. Recent studies also indicate that A20 is an important tumor suppressor that is inactivated in B-cell lymphomas. Zinc suppresses generation of NF-κB-regulated inflammatory cytokines by induction of A20. Zinc sensitizes prostate cancer cells to apoptosis through its inhibition of NF-kB. Zinc supplementation decrease NF-κB activity
Zinc and inflammatory/immune response in aging.
A20: central gatekeeper in inflammation and immunity.
Regulation of NF-κB signaling by the A20 deubiquitinase.
Zinc-suppressed inflammatory cytokines by induction of A20-mediated inhibition of nuclear factor-κB.
Zinc is involved in activation of the p53 that appears to be an important component of the apoptotic process and also in activation of certain members of the caspase family of proteases. p53 is one of the most famous tumor suppressor genes. Absence of p53 expression or expression of mutant p53 (mtp53) are common in human cancers and are associated with increased cancer resistance to chemo- and radiotherapy. It has been demonstrated that p53 contains a tightly bound zinc atom that is necessary for the DNA binding activity of the protein. Zinc has been shown to induce apoptogenesis in prostate cancer cells that results from its direct effect on mitochondrial release of cytochrome c followed by activation of the caspase cascade and ultimately apoptosis. Caspase-6 is the most sensitive apoptosis-related molecular target of zinc. It cleaves and activates the proenzyme form of caspase-3 and is also responsible for the cleavage of lamins and therefore, is directly involved in nuclear membrane dissolution. Zinc has also been shown to inhibit the invasive capabilities of malignant prostate cells.
Zinc can also inhibit absorption of micronutrients, specifically iron and copper. Iron is an essential cellular nutrient that is critical for DNA synthesis, and is required for the growth of all living cells. Cancer cells require more iron than typical healthy cells do in order to support the uncontrolled growth and tumors grow better in an iron-rich environment. This should be taken into consideration when treating cancer patients, and senior citizens who have a high risk of developing cancer. Copper stimulates the growth of blood vessels. Iron chelation may be a useful tool for cancer treatment. Taking large quantities of zinc (50 mg/day or more) over a period of weeks can interfere with iron and copper bioavailability.
Unlike other metals, zinc is virtually nontoxic even at higher doses. The homeostatic mechanisms that regulate its entry into, distribution in and excretion from cells and tissues are so efficient that no disorders are known to be associated with its excessive accumulation in contrast to iron, copper, mercury and other metals. Long-term, high-dose zinc supplementation interferes with the uptake of copper. Hence, many of its toxic effects are in fact due to copper deficiency.
Zinc becomes cytotoxic if its extracellular concentration exceeds the capacity of the zinc homeostatic system. Elevated extracellular zinc concentrations lead to the breakdown of the zinc transporting system of the plasma membrane. The resulting enhanced intracellular zinc concentration activates the apoptosis. Zinc induces apoptosis via deficiency as well as overload. Whether dietary zinc intake affects intraprostatic zinc levels is unknown. However, a therapeutic dose of zinc is 50-100 mg of elemental zinc a day. Remember that elemental zinc is only 30% of the weight of zinc citrate so a 50 mg zinc citrate pill has only 15 mgs of elemental zinc. Herbalzym Anti-Cancer Mineral contains 100% bioavailable (highly absorbable) liquid ionic zinc. Herbalzym uses a proprietary process that transforms pure mineral crystals into a fully hydrated, 100% bioavailable liquid ionic supplement. So it is not naecssary to take large doses in order to be effective. Cancer prevention is easier than you may think, but treatment is another story.
Zinc: a promising agent in dietary chemoprevention of cancer.
Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations.
Novel Chelators for Cancer Treatment: Where Are We Now?
Cancer cell iron metabolism and the development of potent iron chelators as anti-tumour agents.
The essential toxin: impact of zinc on human health.
Comparative absorption of zinc picolinate, zinc citrate and zinc gluconate in humans.