 Quercetin is one of the most ubiquitous flavonoids found in many fruits, vegetables, nuts, and red wine, and exerts anti-inflammatory and anti-carcinogenic activities. Research shows that quercetin may help to treat and prevent colon cancer. Quercetin can affect growth of colon cancer cells by both decreasing polyamine biosynthesis and inducing apoptosis. Polyamines are involved in cell growth and differentiation.
Quercetin is one of the most ubiquitous flavonoids found in many fruits, vegetables, nuts, and red wine, and exerts anti-inflammatory and anti-carcinogenic activities. Research shows that quercetin may help to treat and prevent colon cancer. Quercetin can affect growth of colon cancer cells by both decreasing polyamine biosynthesis and inducing apoptosis. Polyamines are involved in cell growth and differentiation.
The Wnt signaling pathway plays a pivotal role in cellular developmental processes and human carcinogenesis. Inhibition of expression of cyclin D(1) and survivin as well as the Wnt/beta-catenin, p21-RAS signaling pathway could be qualified as promising targets for innovative treatment strategies of colon cancer. Quercetin inhibits these pathways. Moreover, Quercetin activates AMPK (AMP-activated protein kinase), a physiological cellular energy sensor, through ROS generation. Activation of AMPK strongly suppresses cell proliferation in both nonmalignant and tumor cells.
That’s all promising, but it’s too early to recommend quercetin as a supplement. First of all, you have no idea what’s in the bottle you buy. There could be little or no quercetin. Quercetin absorption can vary, depending on its source. Low oral bioavailability of quercetin due to insolubility in water has limited its use as a food additive or dietary supplement. Quercezym™ contains enzymatic fermented high purity quercetin. It has perfect bioavailability and pharmacokinetics of active quercetin. Quercezym™ is the one and only product that has bioavailability of high purity quercetin enough to induce apoptosis in colon cancer cells. Otherwise, quercetin wouldn’t have worked.
Quercetin inhibits human DLD-1 colon cancer cell growth and polyamine biosynthesis.
Apoptotic effect of quercetin on HT-29 colon cancer cells via the AMPK signaling pathway.
Immune system is first line of defence against cancer. Interactions between immune cells and tumor cells involve direct cell-cell physical contact and release of modulator molecules, primarily cytokines and chemokines. In this survival and death battle, on the one hand, the immune cells may prevail and eradicate the tumor cells. On the other hand, tumor cells can counterattack the immune cells by producing inhibitory molecules, activating the immune suppressive cells or acquiring apoptosis-resistant mechanisms to avoid destruction by the immune system. Thus, the anti-tumor immune response can be a two-edged sword and can result in both positive and negative consequences.
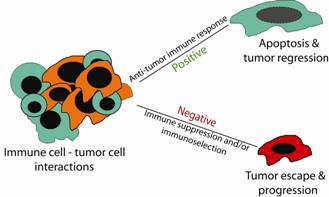 The biologic failure of the immune system to effectively suppress neoplastic disease in immune-competent hosts is not fully understood and has remained a fundamental paradox of tumor immunobiology. Cytotoxic T lymphocytes (CTLs) are the primary effector cells against tumor cells and function primarily through two effector mechanisms. The first cytolytic pathway depends on the polarized secretion of perforin and granzymes. The second effector mechanism involves the interaction of FasL on activated CTLs with its receptor Fas on the target tumor cells. Recent studies have demonstrated that the perforin effector mechanism of tumor-specific CTLs can be selectively suppressed by immune suppressive Treg cells in vivo, whereas acquisition of resistance to Fas-mediated apoptosis is a hallmark of metastatic tumors. Therefore, both the perforin and Fas pathways can be impaired in the tumor microenvironment. Accordingly, suppression of immunosuppression, sensitization of tumor cells to Fas-mediated apoptosis or targeting additional anti-tumor cytotoxic pathways is essential for CTL-based immunotherapy against cancer.
The biologic failure of the immune system to effectively suppress neoplastic disease in immune-competent hosts is not fully understood and has remained a fundamental paradox of tumor immunobiology. Cytotoxic T lymphocytes (CTLs) are the primary effector cells against tumor cells and function primarily through two effector mechanisms. The first cytolytic pathway depends on the polarized secretion of perforin and granzymes. The second effector mechanism involves the interaction of FasL on activated CTLs with its receptor Fas on the target tumor cells. Recent studies have demonstrated that the perforin effector mechanism of tumor-specific CTLs can be selectively suppressed by immune suppressive Treg cells in vivo, whereas acquisition of resistance to Fas-mediated apoptosis is a hallmark of metastatic tumors. Therefore, both the perforin and Fas pathways can be impaired in the tumor microenvironment. Accordingly, suppression of immunosuppression, sensitization of tumor cells to Fas-mediated apoptosis or targeting additional anti-tumor cytotoxic pathways is essential for CTL-based immunotherapy against cancer.
Moreover, a recent study shows that colon cancer cells produce immunoregulatory glucocorticoids and suggests that the synthesis of bioactive glucocorticoids in colon cancer cells may account as a novel mechanism of tumor immune escape. Glucocorticoids (both natural and synthetic) inhibit the immune response, thus preventing inflammation from occurring.
Hepazym™ is an immunomodulator of the potentiator type, which has demonstrated an enhancing effect on the function and number of various cells of the immune system in humans. Hepazym™ is designed to naturally build and provide long term support for healthy immune function and sound immune response, even while suffering from cancer. Hepazym™ has been developed in research on viral pathogenesis and mechanistic insights into viral modulation of immune receptor signaling. Hepazym™ contains the latest research based formula of bio-active fermented 32 naturally-occurring compounds with therapeutic potential for immune system enhancement, modulation or anti-cancer activity.
Hepazym™ helps to maintain resistance to infection and defend against cancer. Hepazym™ has been independently tested in a ground-breaking international clinical trial with excellent results. Hepazym™’s multi strain probiotic formulations are also recommended because each person’s intestinal flora has a unique bacterial community (very similar to fingerprints). Metabolic effects of probiotics on the intestinal epithelium include production of short chain fatty acids which influence epithelial cell metabolism, turnover and apoptosis. Probiotics, through secreted molecules, influence the innate inflammatory response of epithelial cells to stimuli from the gut lumen, and reduce mucosal inflammation. Through effects on dendritic, and possibly epithelial, cells they influence naïve T cells in the lamina propria of the gut and thus influence adaptive immunity. Hepazym™ the ultimate high potency, broad-spectrum probiotic formula, providing a 16 billion live cell count per day of 17 species of beneficial cultures including soil-based probiotics. Hepazym™ is the most effective supplement for anti-cancer and immune system building. No other supplement shows the quick and consistent benefits that Hepazym™ does.
Role of apoptosis resistance in immune evasion and metastasis of colorectal cancer.
Colon cancer cells produce immunoregulatory glucocorticoids.
Roles for inflammation and regulatory T cells in colon cancer.
Antitumor activity and immune response induction of a dual agonist of Toll-like receptors 7 and 8.
The application of probiotic fermented milks in cancer and intestinal inflammation.
Polyamines are involved in cell growth and differentiation. Ornithine decarboxylase (ODC), a key enzyme in mammalian polyamine biosynthesis, has been proposed to be a marker of colonic epithelial cell proliferation and risk for colorectal cancer. ODC regulates cell division by catalysing the first step in polyamine biosynthesis. Apigenin is a nonmutagenic bioflavonoid which is presented in leafy plants and vegetables (e.g., parsley, artichoke, basil, celery) and has significant chemopreventive activity. Apigenin is a major constituent of chamomile. Research shows that it may reduce ODC activity; inhibit cancer cell signal transduction and induce apoptosis. Apigenin is almost insoluble in water (pH = 6.6). Apizym™ is the one and only product that has perfect solubility, bioavailability of high purity apigenin.
Ornithine decarboxylase gene is overexpressed in colorectal carcinoma.
Molecular targets of apigenin in colorectal cancer cells: involvement of p21, NAG-1 and p53.
Apigenin acts on the tumor cell invasion process and regulates protease production.
Eflornithine is a drug which was initially developed as a cancer medication. It is also an effective hair growth inhibiting agent. As a topical application, the drug has been shown to be an effective hair growth retardant in some patients, and is sold under the brand name Vaniqa. Protein polyamines are needed for rapid cell growth and differentiation, and the production of these polyamines depends on the activity of enzyme ornithine decarboxylase (ODC). Eflornithine is believed to block ODC and thereby slow the growth and differentiation of the cells within the hair follicles.
A trial was reported involving combination chemoprevention with eflornithine and NSAID sulindac treatment on colorectal adenoma recurrence. Sulindac, like other NSAIDs, has polyamine-inhibitory properties via induction of spermidine spermine acetyltransferase (SSAT) and resultant cellular polyamine export. Together, eflornithine and sulindac decrease the cellular polyamine pools. Results from a recent trial shows that sulindac combined with eflornithine suppresses colorectal carcinogenesis.
Polyamines as mediators of APC-dependent intestinal carcinogenesis and cancer chemoprevention.
Sulindac is a general Cox enzyme inhibitor. It’s an inexpensive prescription drug that has been shown to both prevent and treat colon cancers through Cox-2 dependent and independent mechanisms. Clearly, sulindac has anti-cancer properties that transcend its ability to inhibit the Cox-1 and Cox-2 enzymes.
Phosphodiesterase 5 (PDE5) is an enzyme that accepts cGMP and breaks it down. Sildenafil, vardenafil and tadalafil are inhibitors of this enzyme, which bind to the catalytic site of PDE5. PDE5 is expressed in human colonic cells and in intestinal tissue and its activity is regulated by intracellular cGMP levels in these cells that increase on GCC (guanylyl cyclase C) activation. PDE5 was found to be overexpressed in colon tumor cell lines as well as in colon adenomas and adenocarcinomas compared with normal colonic mucosa. PDE5 inhibition, cGMP elevation, and inhibition of β-catenin transcriptional activity may contribute to the chemopreventive properties of sulindac.
AKT kinases play a key role in proliferation, cell survival, and tumorigenesis. Sulindac can inhibit the AKT kinase. Actually, a metabolite of sulindac, sulindac sulfide, is the AKT inhibitor. It appears that sulindac metabolites inactivate AKT by two different pathways. The first pathway degrades AKT by the caspase-8 enzyme. The second pathway decreases phosphorylation of the AKT kinase. Sulindac also promotes the synthesis of p21Waf1. p21Waf1, P53 and cyclin D1 belong to the cell cycle-regulating family of proteins, and the loss of activity of proteins p21Waf1 and P53 seems to be one of the most important regulatory mechanisms of carcinogenesis in colorectal cancer.
Sulindac suppresses beta-catenin expression in human cancer cells.
NSAID sulindac and its analog bind RXRalpha and inhibit RXRalpha-dependent AKT signaling.
Polyethylene glycol (PEG) is a nonabsorbed, nonmetabolized, water-soluble polymer that has been used to treat constipation in France since 1996 and was recently approved for use as a laxative in the US and Canada. Studies demonstrated PEG to be a highly effective chemopreventive agent against colon cancer. PEG induced p21Waf1 levels back to normal. These results demonstrate that PEG is involved in p21 regulation concomitant with G1S phase cell cycle arrest and it is through these effects that it can exert its anti-proliferative and hence chemopreventive role. Moreover, PEG induced expression of pro-apoptotic proteins and there was a dramatic and specific induction of apoptosis.
Cytostatic effect of polyethylene glycol on human colonic adenocarcinoma cells.