The war on cancer, fought for four decades marked by failure and frustration. We’ve learned enormous amounts about the disease, but it hasn’t translated into therapy. This is especially true in cancer, where old and new targeted therapies continue to fail and the most recent ones do not offer much improvement on clinical outcome parameters. New drugs are continuously designed and tried, it seems inevitable that genetic and signal protein targets pose too broad flexibility and variability, often changing target characteristics and thus escape treatments turning “magic bullets” into rather “wondering bullets”.
Metabolic modulation may be a viable therapeutic approach in the treatment of cancer and leukemia. Metabolic targeted therapies are aimed at control points of the metabolic network by targeting particular enzymes of major macromolecule synthesis pathways in cancer and leukemia. Solid tumors develop resistance to cell death, in part as a result of a switch from mitochondrial oxidative phosphorylation to cytoplasmic glycolysis. This metabolic remodeling is accompanied by mitochondrial hyperpolarization.
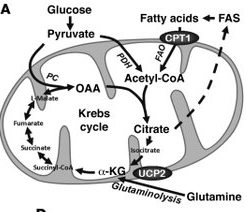 However, recent research has indicated that in the process of generating energy, leukemia cells use a cellular pathway known as fatty acid oxidation (FAO), rather than pyruvate oxidation, as had been previously thought. Therefore, fatty acid oxidation (FAO) is essential for human leukemia cell survival and inhibitors of fatty acid oxidation (FAO) might provide a new approach to treat leukemias.
However, recent research has indicated that in the process of generating energy, leukemia cells use a cellular pathway known as fatty acid oxidation (FAO), rather than pyruvate oxidation, as had been previously thought. Therefore, fatty acid oxidation (FAO) is essential for human leukemia cell survival and inhibitors of fatty acid oxidation (FAO) might provide a new approach to treat leukemias.
The researchers found that inhibition of fatty acid oxidation (FAO) with either etomoxir, a drug that was tested in clinical trials for the treatment of heart disease but never made it to market due to unacceptable toxicities, or ranolazine, a drug approved for the treatment of chronic angina, inhibited human leukemia cells proliferation in vitro. Fatty acid oxidation (FAO) regulates the activity of Bak-dependent mitochondrial permeability transition. Importantly, etomoxir decreased the number of quiescent leukemia progenitor cells in approximately 50% of primary human acute myeloid leukemia samples and, when combined with a chemotherapy agent (cytosine arabinoside), provided substantial therapeutic benefit in a murine model of leukemia. The results support the concept of fatty acid oxidation (FAO) inhibitors as a therapeutic strategy in hematological malignancies.
Angina is chest pain or discomfort that occurs when an area of the heart is deprived of oxygen. Typically, angina is described as a “pressure” or “squeezing” pain that starts in the center of the chest and may spread to the shoulders or arms, the neck, jaw or back. It is usually triggered by extra demand on the heart: exercises, an emotional upset, exposure to cold, digesting a heavy meal are common examples. Cardiac muscle has an extremely rapid rate of metabolism. Blood flow and oxygen consumption are high and proportional to the rate of formation of ATP in the mitochondria. To meet the high demands of energy, the heart must produce a constant and plentiful supply of ATP, energy that is derived from the metabolism of fatty acids and carbohydrates. Fatty acids are the preferred substrate of the myocardium, contributing approximately 60–80% of the energy for ATP synthesis.
In fact, the failing heart has often been described as ‘energy starved’. Several studies have shown reduced ATP content and dysfunctional mitochondria in the failing heart while others have shown increased fatty acid oxidation and decreased carbohydrate oxidation in patients with heart failure compared with normal age-matched healthy volunteers.
Fatty acid oxidation (FAO) strongly inhibits pyruvate oxidation in the mitochondria and the uptake and oxidation of glucose. Partial inhibition of fatty acid oxidation increases glucose and pyruvate oxidation and decreases lactate production, resulting in higher pH and improved contractile function during ischaemia. Trimetazidine and Ranolazine are a partial fatty acid oxidation (FAO) inhibitor which shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation. Since the oxidation of glucose requires less oxygen than the oxidation of fatty acids, fatty acid oxidation (FAO) inhibitors can help maintain myocardial function in times of ischemia. Other possible mechanism by which fatty acid oxidation inhibitors could act is by reducing the formation of reactive oxygen species (ROS) and improves reperfusion mechanical function. It might be possible to translate this approach to the clinic as a therapeutic strategy to combat leukemia.
Anti-anginal effects of partial fatty acid oxidation inhibitors.
Ranolazine: a new approach to management of patients with angina.
However, most inhibitors of fatty acid oxidation (FAO) are activating ligands of PPARs (peroxisome proliferator-activated receptors). PPARs promote leukemia cells survival. PPARs are members of the nuclear hormone receptor super-family, regulating gene expression via their ligand-activated transcriptional activity. There are three known subtypes of PPARs (alpha, beta/delta and gamma). Human PPAR gamma was first cloned from the bone marrow, and the receptor is expressed in monocytes/macrophages as well as in B and T lymphocytes and bone marrow precursors. PPARs play an important role in lipid metabolism, insulin sensitization, and cancers in addition to controlling the expression of many genes involved in lipid metabolism, and insulin sensitization. PPARs control myocardial metabolism by transcriptionally regulating genes encoding enzymes involved in fatty acid and glucose utilization.
The potential role of PPAR in cancer tumor formation in humans is controversial. While two studies implicated PPAR gamma in promotion and development of cancer tumor, another report indicated a possible protective role for PPAR gamma agonists against cancer tumor. L-aminocarnitine, a carnitine analog, inhibits fatty acid oxidation (FAO) efficiently, but does not activate PPARs. L-aminocarnitine inhibits carnitine palmitoyltransferase (CPT) with different sensitivities towards CPT1 and CPT2, as well as carnitine acylcarnitine translocase (CACT).
Fatty acids are oxidized inside the mitochondrial matrix but the fatty acids to be oxidized come from the cytosol. Fatty acids are activated in the cytosol by esterification with Coenzyme A (CoA) to form acyl-CoA (RCO-CoA, where R is the fatty acid acyl group). Activated medium-chain fatty acids (C8 and C10) freely diffuse into mitochondria to be oxidized but long chain fatty acids do not diffuse into mitochondria so they must be transported in. The transport of long chain fatty acids into mitochondria for oxidation is accomplished by the carnitine palmitoyltransferase system (CPT1 and CPT2). CPT1 exchanges carnitine for the CoA attached to long chain fatty acids to form a fatty acid-carnitine conjugate (RCO-carnitine). The fatty acid-carnitine is transported into the matrix by a transporter protein in the inner mitochondrial membrane. Once the fatty acid-carnitine is inside the matrix, CPT2 exchanges CoA for carnitine to produce fatty acid-CoA once again, ready to enter fatty acid oxidation in the matrix to produce energy. The free carnitine is transported back out to renew the cytoplasmic pool of carnitine and allow the transfer process to continue.
Characterization of L-aminocarnitine, an inhibitor of fatty acid oxidation.
Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects.
Malonyl coenzyme A (CoA) regulates fatty acid oxidation by inhibiting mitochondrial uptake of fatty acids. Malonyl CoA decarboxylase (MCD) is involved in the decarboxylation of malonyl CoA to acetyl CoA. Therefore, inhibition of Malonyl CoA decarboxylase (MCD) decreases fatty acid oxidation (FAO), secondary to increasing malonyl CoA levels, and increase in glucose oxidation rates. Malonyl-CoA is a potential mediator of cytotoxicity induced by fatty-acid synthase (FAS) inhibition in cancer cells. It is found that inhibition of fatty acid synthase (FAS) using natural agents such as SinnolZym markedly increase the malonyl-CoA levels in cancer cells. It is believed that the increased malonyl-CoA level is responsible for the antitumor activity of these fatty acid synthase (FAS) inhibitors. Regulating malonyl-CoA levels using MCD inhibitors thus constitutes a valuable therapeutic strategy for the treatment of cancer and leukemia.
Malonyl-CoA decarboxylase inhibition as a novel approach to treat ischemic heart disease.
Malonyl CoA control of fatty acid oxidation in the ischemic heart.
Evaluation of inhibition of fatty acid synthase by ursolic acid: positive cooperation mechanism.
Fatty acid synthase inhibition triggers apoptosis during S phase in human cancer cells.
Evaluation of the inhibitory activities of aceraceous plants on fatty acid synthase.