Cancer was formerly believed to be a localized disease, characterized by a lesion, usually in the form of a growth, which appeared at some specific part of the body. This localized lesion was thought to be the result of activity produced by an invading virus, carcinogenic agent, or some form of trauma. Today, there is a growing conviction that cancer is a complex disease that is the end result of a disturbed metabolism. The frequent recurrence of a malignancy after treatment with the conventional methods of surgery, radiation and/or chemotherapy results because the basic underlying metabolic cause of the cancer is rarely considered and consequently remains uncorrected.
In 2007, researchers at the University of Alberta, Canada, reported that DCA (dichloroacetate) has seemingly remarkable anticancer properties. DCA is a tiny molecule, odorless, colorless, inexpensive and relatively non-toxic and researchers think it may soon become an effective treatment for several cancers including lung, breast, and brain tumors.
DCA blocks an enzyme (PDK) in mitochondria — the energy-production centres in cells — causing more glucose to be metabolized in the mitochondria rather than by a different pathway in the cytoplasm. DCA is an inhibitor of mitochondrial pyruvate dehydrogenase kinase (PDK), which inhibits pyruvate dehydrogenase (PDH), a gatekeeping enzyme for the entry of pyruvate into the mitochondrial tricarboxylic acid (TCA) cycle. In mice, DCA treatment appears to reactivate mitochondrial respiration in tumor cells, induces their selective killing, and suppresses cancer growth. DCA has been in clinical trials for years as a treatment for certain mitochondrial diseases, but it has not yet been approved.
Metabolic targeting as an anticancer strategy: dawn of a new era?
Metabolic modulation of glioblastoma with dichloroacetate.
Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer.
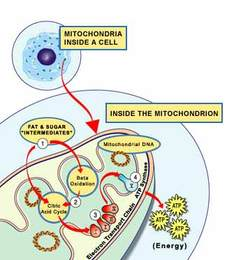 Mitochondria also control cell suicide (apoptosis), and cancer cells are suppressing these cellular structures to prevent the cells from dying, But until recently, it was believed that cancer-affected mitochondria are permanently damaged because of, not the cause of, the cancer, and so these observations provide intriguing insights into the plasticity of tumor metabolism that may offer new opportunities for therapeutic intervention. The important point is that people are now paying more attention to the metabolic control of cancer.
Mitochondria also control cell suicide (apoptosis), and cancer cells are suppressing these cellular structures to prevent the cells from dying, But until recently, it was believed that cancer-affected mitochondria are permanently damaged because of, not the cause of, the cancer, and so these observations provide intriguing insights into the plasticity of tumor metabolism that may offer new opportunities for therapeutic intervention. The important point is that people are now paying more attention to the metabolic control of cancer.
Cancer metabolism is controlled by HIF-1 (hypoxia inducible factor-1), and AKT, the master biochemical switch for growth and survival and cancer and normal cells. HIF-1 is an important regulator of the tumor response to hypoxia, including increased angiogenesis, glycolytic metabolism, and resistance to apoptosis. HIF-1 regulates the transcription of many genes involved in key aspects of cancer biology, including immortalization, maintenance of stem cell pools, cellular differentiation, genetic instability, vascularization, metabolic reprogramming, autocrine growth factor signaling, invasion/metastasis, and treatment failure. HIF-1 expression is elevated in virtually all solid tumors.
HIF-1 is synthesized and degraded on a continuing basis in the presence of oxygen. When oxygen levels decrease (hypoxia), the HIF-1 protein becomes stabilized and increases in the cell. To survive in hypoxic environments, organisms must be able to cope with redox imbalance and oxygen deficiency. When HIF-1 enters the nucleus, it stimulates the synthesis of over 100 proteins including glycolytic enzymes, glucose transporters and VEGF, vascular endothelial growth factor. This promotes glycolysis and the production of ATP in the relative absence of oxygen. VEGF stimulates the growth of blood vessels into organs thereby increasing the delivery of addition oxygen and nutrients, such as glucose.
HIF-1-regulated glucose metabolism: a key to apoptosis resistance?
Oltipraz (5-[2-pyrazinyl]-4-methyl-1,2-3-thione) is originally developed more than 40 years ago as an antischistosomal (parasite killing) agent, it was found to protect against chemically induced carcinogens in the lung, stomach, colon, and urinary bladder in animals. Oltipraz inhibits HIF-1 activity and HIF-1 dependent tumor growth, which may result from a decrease in HIF-1 stability through S6K1 inhibition in combination with an H(2)O(2)-scavenging effect.
Oltipraz: a laboratory and clinical review.
Oltipraz is also an anticarcinogen (stomach, skin, bladder, breast, colon, pancreas, lung, liver) and proposed antimutagen. It inhibits carcinogenesis induced by polycyclic aromatic hydrocarbons and N-nitrosamines — agents that constitute some of the carcinogenic components of tobacco. Since bladder cancer is more than twice as common in smokers as in nonsmokers, oltipraz may be useful in preventing the development of cancer in smokers. Oltipraz is unique in its dual capacity as an antischistosomal and anticarcinogenic agent. Its chemopreventive abilities can be effective in patients with histories of Schistosoma haematobium (parasitic blood fluke) bladder infections, who are at increased risk for developing bladder cancer. This is more common in tropical than in temperate climates.In 1995, Oltipraz has been evaluated in residents of Qidong, China, who are at high risk for exposure to aflatoxin and development of hepatocellular carcinoma.
Role of phase 2 enzyme induction in chemoprotection by dithiolethiones.
Mechanistically, zinc ion inhibits HIF-1 activation
Inhibitory effect of zinc on hypoxic HIF-1 activation in astrocytes.
Cancer cells, unlike ordinary cells, are capable of growing in multiple layers that ordinarily make it hard for oxygen to reach cells located in the core. To overcome this form of hypoxia, cancerous growth triggers HIF-1 to make more blood vessels that feed oxygen to the tumor and keep it alive. New study shows that the methylation of the Reptin protein inhibits the HIF-1 and can cause the cancer cell to suffocate. Reptin is a subunit of different chromatin-remodeling complexes. Proteins can be enzymatically modified in several ways by the addition of methyl groups from S-adenosylmethionine. Reactions forming methyl esters on carboxyl groups are potentially reversible and can modulate the activity of the target protein. The scientist said that by checking the natural level of Reptin protein methylation in a body, scientists may be able to detect cancer growth in early stages. If cancer grows in the body, the Reptin protein shoots up to 10 times normal levels.
Negative regulation of hypoxic responses via induced Reptin methylation.
AKT (protein kinase B) is the master switch for the development of all cancers and leukemias. If we can inhibit the activation of this enzyme, cancer/leukemia will cease to exist. This is not an exaggerated statement.The Akt is involved in various cellular processes including cell proliferation, growth and metabolism. Hyperactivation of Akt is commonly observed in human tumours. However, Akt also plays an essential role in other physiological processes, such as the insulin-regulated transport of glucose into muscle and fat cells. This process, which is essential for whole body glucose homeostasis in mammals, is thought to be mediated via Akt-dependent movement of GLUT4 glucose transporters to the plasma membrane. Aerobic glycolysis is fundamentally important in the metabolism of all cancer and leukemia cells. AKT also promotes aerobic glycolysis by the activation of specific enzymes. The inhibition of glycolysis induces the rapid apoptosis of cancer cells. AKT is indeed a master growth and survival switch in all cancers and leukemias.
Is Akt the “Warburg kinase”?-Akt-energy metabolism interactions and oncogenesis.
Metabolic regulation of Akt: roles reversed.
Akt inhibitors reduce glucose uptake independently of their effects on Akt.
Synthetic phospholipid drug called Perifosine inhibits Akt activation. Perifosine is an orally active alkyl-phosphocholine compound with potential antineoplastic activity. Targeting cellular membranes, perifosine modulates membrane permeability, membrane lipid composition, phospholipid metabolism, and mitogenic signal transduction, resulting in cell differentiation and inhibition of cell growth.
Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation.
In vitro and in vivo inhibition of neuroblastoma tumor cell growth by AKT inhibitor perifosine.